Manufacturing for the Healthcare Sector

From the moment a patient commences medical treatment to the moment he or she can be declared cured, a number of things need to happen: From diagnostics to therapy up to the completion of rehabilitation – each of these steps requires biomedical or medical technology products and devices. Doctors, pharmacists, and nursing staff must be able to trust that the materials themselves and their finish are of the highest quality. Even tiny errors in the manufacturing of medical products can have life-threatening consequences.
It is precisely because of these exacting standards that medical engineering is among the most innovative industries. Whether it is less invasive operational procedures, more reliable implants, or more effective vaccines – new technologies and products increase our quality of life and help save lives. The current patent ranking of the European Patent Office highlights the innovative strength of the MedTech industry: It ranks first in terms of patent applications, ahead of digital communications and computer technologies. Equally remarkable is the dynamic momentum with which these innovations are taking place: According to BVMed, the German Medical Technology Association, German medical engineering manufacturers generate approximately one third of their sales from products that are less than three years old. Manufacturers also work extremely closely and transparently with users from the very beginning. In addition, in 52 percent of cases, it is doctors and nursing staff who provide the inspiration for the development of new products.
Rapidly putting new technologies into use in real-world applications without compromising on safety and effectiveness is a constant challenge for the industry. Medical products must pass extensive technical checks before they are tested in clinical trials and approved for patients. This involves testing to determine whether the products’ characteristics – e.g., density, strength, compatibility, sterility – meet the performance requirements that make their medical use possible in the first place.
Hence, without engineering research – no progress in medical technology. This applies in particular to the three major industry trends: miniaturization, microfluidics and digitalization.
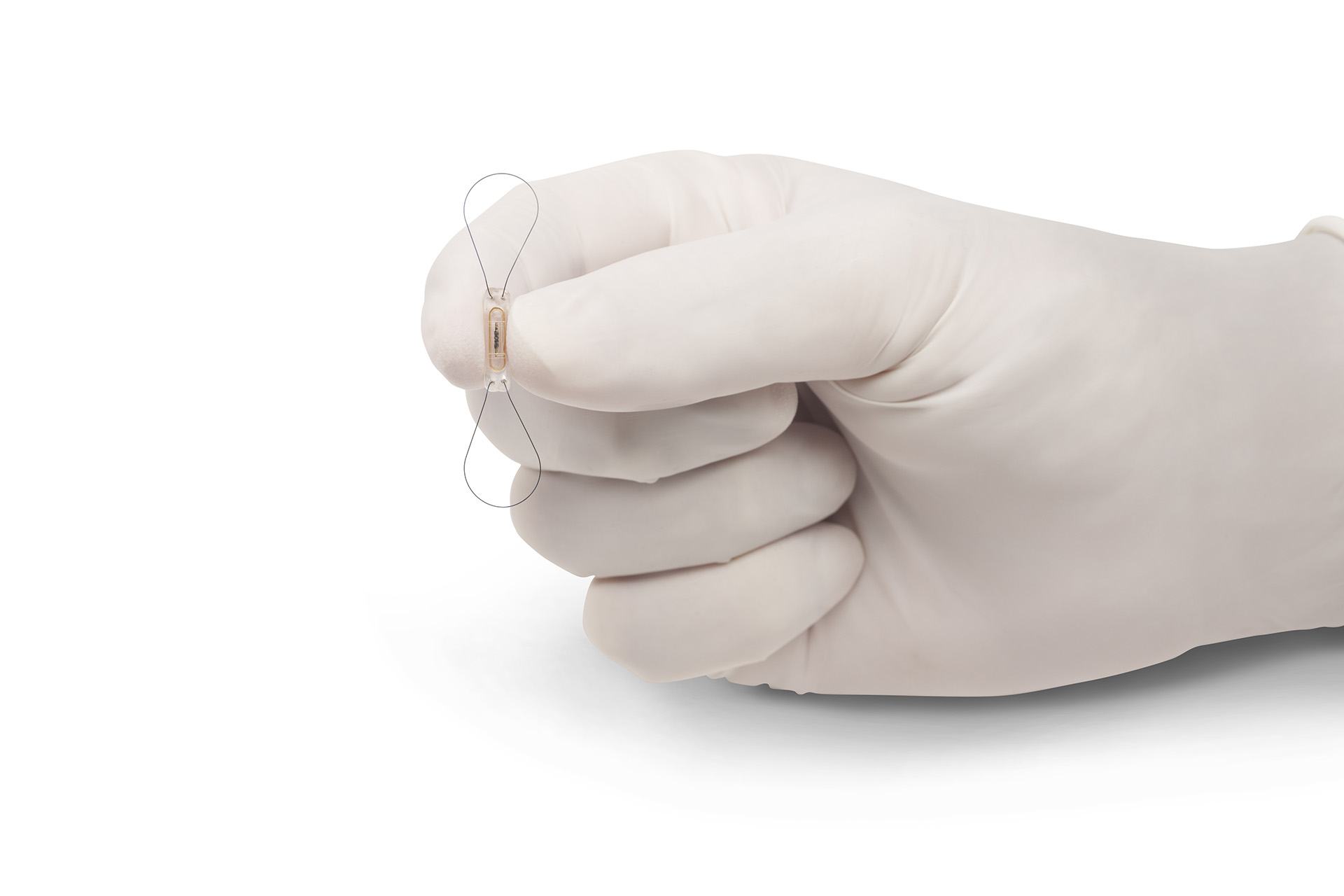
Miniaturization: Maximum precision for the smallest products
Medical disciplines such as cardiology, surgery, radiology and diagnostics currently rely on complex, miniaturized products. At the Production Technology Center (PTZ) Berlin, interdisciplinary teams consisting of mechanical engineers, biotechnologists and electrical engineers are conducting research on how they can be manufactured safely and efficiently. Using ultra- and high-precision processes alongside nano- and biotechnologies, they are for instance able to replicate structures for fluid mixing systems or cell separations.
Our researchers are also improving quality of life for people with prosthetic joints: They optimize materials and manufacturing processes in such a way that they obtain an improved stress state, thus enabling a longer service life for prostheses. In addition to conventional materials such as metals and plastics, scientists are also taking materials that are difficult to work with, such as metallic glass and refractory metals, and making them compatible for medical applications. They are, for example, developing ideal surfaces to prevent accumulation of microorganisms or rejection reactions.
Microfluids: New scaling potential for pharmaceutical manufacturers
Fluid-conveying systems are widely used in real-world medical applications, such as in lab-on-a-chip systems for point-of-care diagnostics. Researchers at the PTZ are developing special manufacturing technologies for producing such microfluidic chips. In their own research, they are using chips to optimize the enveloping of mRNA molecules in lipid nanoparticles – an important step in scaling up the production of vaccines against the corona virus.
Another aspect of vaccine production: Currently, vaccines based on cell and gene therapy are still being produced laboriously in manual batch-based production processes. However, fully automated and integrated end-to-end processes could soon revolutionize not just vaccine production, but pharmaceutical production as a whole. The Fraunhofer »Pharmaceutical Production 4.0« initiative is therefore developing innovative concepts for the automation, digitalization, and modularization of pharmaceutical production and quality assurance of drugs. Application scenarios include not only vaccines, but also cell therapeutics and stem cells for oncology and regenerative medicine, as well as for the treatment of immune and infectious diseases. The team of researchers at Fraunhofer IPK, with its expertise in production and material technologies, as well as actuators and sensors, is working primarily on the parallelization of manufacturing processes and on facilitating high-throughput processes. This could drastically reduce development and production cycles in the future.
Digitalization: Better diagnoses and therapies
Nearly every aspect of today’s world requires digitalization to function – and medicine is no exception. Whether it is biosensors for blood glucose measurements, camera chips for retinal implants, or AI-supported imaging processes for surgery – digital technologies have become indispensable in healthcare. They allow medical professionals to detect diseases earlier and administer treatments more efficiently – i.e. more individually tailored to the patient – as well as to optimize follow-up treatments, including rehabilitation and care.
However, digital solutions in medicine have even greater potential applications. Since 2016 and embedded in the German federal government’s High-tech Strategy 2025 and Digital Agenda, the German Federal Ministry of Education and Research has been funding new developments in the field of medical engineering, above all in the areas of medical informatics, big data, as well as digital diagnosis and therapy methods. eHealth, telemedicine, and telemonitoring play a key role in this regard, both for healthcare and for medical technology companies. While surgeons in future could be performing minimally invasive surgery with Augmented Reality (AR), or patients with spastic movement disorders treated with the aid of Virtual Reality (VR), context-sensitive assistance may support manufacturers with their highly specialized production processes. Here, AR and VR solutions could help ensure the zero-error strategy required in medical technology production, as well as guide specialists through individual process steps in a targeted manner that is tailored to their respective level of training.
The prerequisite for this is effective process monitoring. Scientists at PTZ Berlin are therefore applying longestablished condition monitoring strategies from mechanical and plant engineering to medical engineering. But this transfer of strategies is not a one-way street – conversely, they are also utilizing technologies originally developed for medical applications in the production halls of injection molding manufacturers and automotive suppliers. One such example involves imaging processes such as computer tomography. In high-throughput processes for manufacturing electric vehicle components, an inline CT now provides seamless documentation of all processing steps, thereby ensuring reliable and stable process and component monitoring.
 Fraunhofer Institute for Production Systems and Design Technology
Fraunhofer Institute for Production Systems and Design Technology