Interview with Dirk Michels, Abiomed Europe GmbH
The smallest heart pump in the world comes from Germany. The Impella heart pump is used in emergency medicine and for treating coronary heart diseases and heart failure. The purpose of Impella heart pumps, which can be used minimally invasively or surgically, is to support and relieve strain on the heart, regenerate cardiac function, and allow patients to enjoy an improved quality of life. We spoke with Dirk Michels, Vice President Global Manufacturing & Supply Chain and Managing Director Abiomed Europe Operations at the manufacturer Abiomed, about the importance of modern medical technology solutions and how findings from research and development can be rapidly and safely used in applications for real-world care.
futur: Heart failure and coronary artery disease are the leading causes of death in both men and women. How do your products and technologies aid in the treatment
of heart disease?
Michels:
Our heart pumps are used during complex coronary interventions and in emergency medicine. Around the world, more than 170,000 patients have been treated with our small Impella heart pumps.
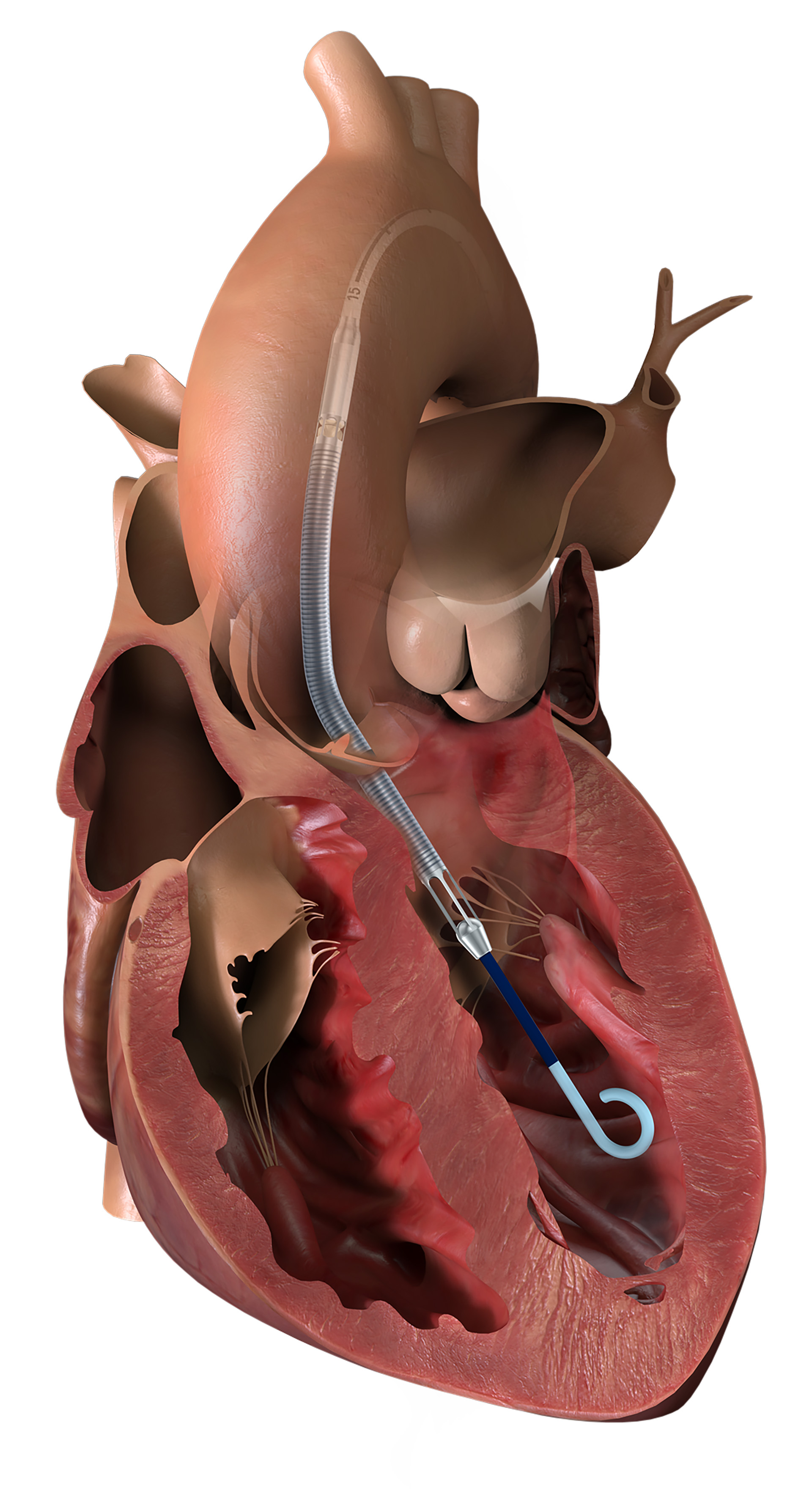
The Impella heart pump restores heart function by being inserted minimally invasively into the human heart via the femoral or shoulder artery and, depending on the pump type, temporarily taking over all or part of the heart’s pumping function, such as during a percutaneous coronary intervention. Hence, our heart pumps make the intervention safer and more effective in high-risk patients.
In emergency medicine, in the event of a heart attack, for example, the Impella heart pump enables cardiac recovery in shock patients. Our heart pumps aid with blood circulation, stabilize hemodynamics in patients, and improve the blood supply to end organs. As a result, they are able to promote regeneration of the heart muscle and thus improve quality of life for patients.
futur: Ever smaller, smarter and digitally networked – this is what Impella technology claims to be. What challenges does this pose for manufacturing and production?
Michels:
High-tech engineering is where we are at home. Manufacturing of the Impella heart pump requires compliance with the highest standards – from the supply chains and the materials used to the actual production and the final inspection. This requires maximum performance from our employees each day and can only succeed thanks to long-standing experience, technical expertise, and quality management. The latest technology in our product portfolio is the world’s smallest heart pump with a diameter of 3 mm – the Impella ECP heart pump, which, by the way, is »Made in Berlin«.
futur: Apart from your product lines you also provide service platforms with SmartAssist and Impella Connect. What is the motivation behind this and how have patients and physicians responded to these offerings?
Michels:
SmartAssist, our most recent platform-based innovation, provides concrete advantages for treatment: For example, SmartAssist sensor technology can be used to make a necessary correction in the position of the Impella heart pump even without imaging and relieve stress on the heart more effectively during treatment.
Our new Impella Connect technology, which is currently being introduced in German hospitals, is a cloud-based platform that enables physicians to provide patients using our Impella heart pumps with even better care. With Impella Connect technology, patients’ treatment and recovery progress can be monitored and controlled online, 24 hours a day, 7 days a week, from any internet-enabled mobile device.
futur: Medical devices are required to undergo extensive technical testing and clinical trials before they can be used on patients. How do you ensure that your latest developments are brought to market quickly and safely?
Michels:
We have decades of experience in translating innovative technologies into safe, market-ready products. Apart from our own R&D department, we also invest in clinical research in order to achieve the best possible results for our patients. We have now completed seven U.S. Food and Drug Administration (FDA) studies and five post-market approval studies which demonstrate the benefits of Impella heart pumps.
futur: What does the future of cardiac medicine look like? Which products and technologies will become necessary?
Michels:
We see a great deal of opportunity for users and patients where telemedicine and digitalization are concerned, because with the targeted use of these technologies, we will be able to continuously improve treatment outcomes for patients.
futur: The corona virus pandemic has shown us all how important medical technology products and processes are for our health, life, and quality of life. To what extent do you notice this increased appreciation in your company, as well as the effects of the crisis?
Michels:
Our focus has always been on the patient. That is exactly why we at Abiomed have dedicated ourselves entirely to the patients’ well-being in everything we do and all the initiatives we launch. Our solutions are specifically designed to further improve individually tailored patient treatments, which has become necessary as a result of the Corona pandemic. Because that is ultimately our goal – focusing on patients and helping them in the best possible manner.
 Fraunhofer Institute for Production Systems and Design Technology
Fraunhofer Institute for Production Systems and Design Technology